- Risk factors for SARS-CoV-2 transmission during a movie theater outbreak in Incheon in the Republic of Korea, November 2021: a retrospective study
-
Hye Young Lee, Young-Joon Park, Sang-Eun Lee, Han-Na Yoo, Il-Hwan Kim, Jin Sun No, Eun-Jin Kim, Jungyeon Yu, Sanghwan Bae, Mi Yu
-
Osong Public Health Res Perspect. 2024;15(1):45-55. Published online January 31, 2024
-
DOI: https://doi.org/10.24171/j.phrp.2023.0269
-
-
 Graphical Abstract Graphical Abstract
 Abstract Abstract
 PDF PDF
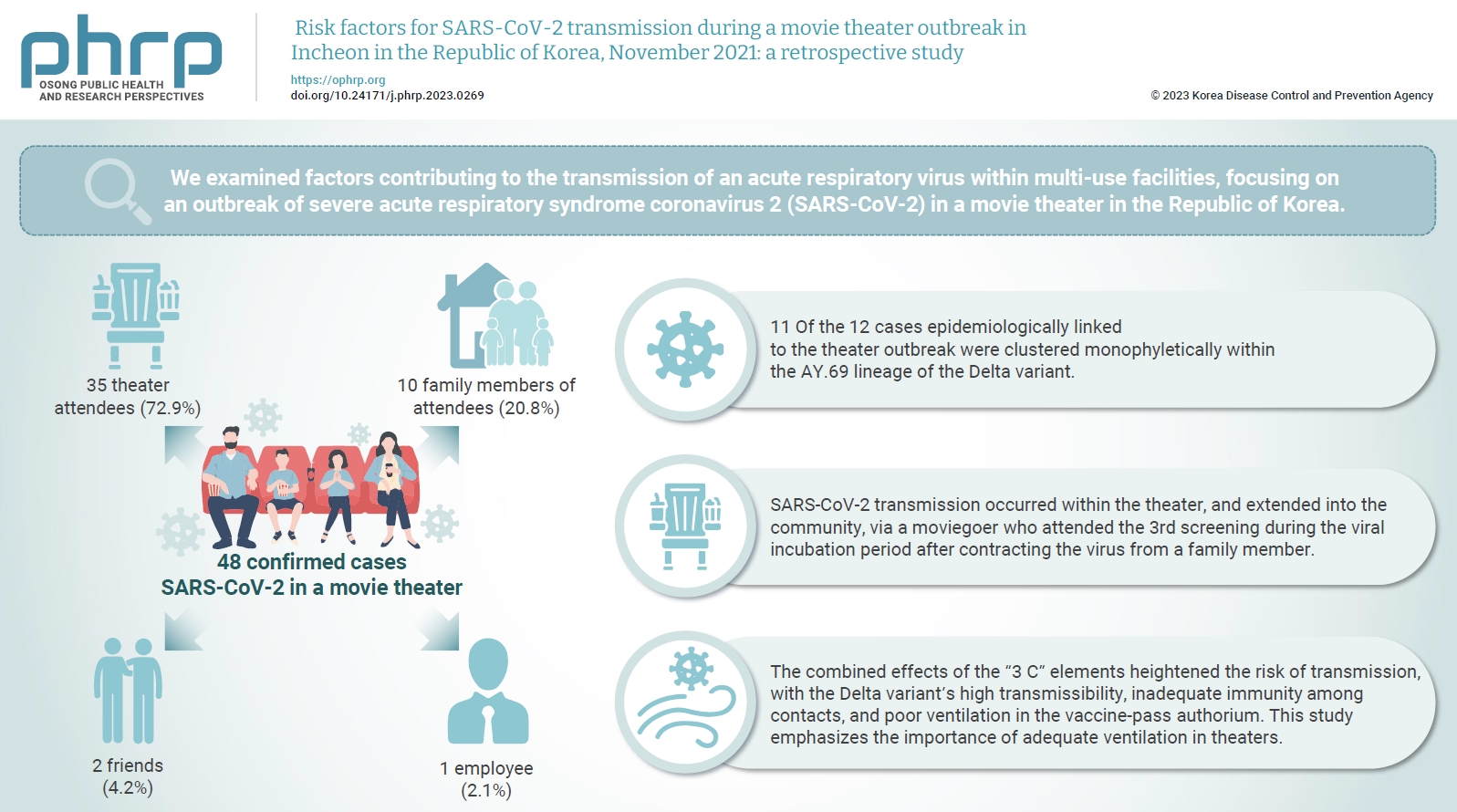 - Objectives
We examined factors contributing to the transmission of an acute respiratory virus within multi-use facilities, focusing on an outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a movie theater in the Republic of Korea. Methods: This retrospective cohort study involved a descriptive analysis of 48 confirmed cases. Logistic regression was applied to a cohort of 80 theater attendees to identify risk factors for infection. The infection source and transmission route were determined through gene sequencing data analysis. Results: Of the 48 confirmed cases, 35 were theater attendees (72.9%), 10 were family members of attendees (20.8%), 2 were friends (4.2%), and 1 was an employee (2.1%). Among the 80 individuals who attended the 3rd to 5th screenings of the day, 35 became infected, representing a 43.8% attack rate. Specifically, 28 of the 33 third-screening attendees developed confirmed SARSCoV-2, constituting an 84.8% attack rate. Furthermore, 11 of the 12 cases epidemiologically linked to the theater outbreak were clustered monophyletically within the AY.69 lineage. At the time of the screening, 35 individuals (72.9%) had received 2 vaccine doses. However, vaccination status did not significantly influence infection risk. Multivariate analysis revealed that close contacts had a 15.9-fold higher risk of infection (95% confidence interval, 4.37–78.39) than casual contacts. Conclusion: SARS-CoV-2 transmission occurred within the theater, and extended into the community, via a moviegoer who attended the 3rd screening during the viral incubation period after contracting the virus from a family member. This study emphasizes the importance of adequate ventilation in theaters.
- Results of contact tracing for SARS-CoV-2 Omicron sub-lineages (BA.4, BA.5, BA.2.75) and the household secondary attack risk
-
Mi Yu, Sang-Eun Lee, Hye Young Lee, Hye-jin Kim, Yeong-Jun Song, Jian Jeong, Ae Kyung Park, Il-Hwan Kim, Eun-jin Kim, Young-Joon Park
-
Osong Public Health Res Perspect. 2023;14(3):173-179. Published online June 22, 2023
-
DOI: https://doi.org/10.24171/j.phrp.2022.0285
-
-
 Graphical Abstract Graphical Abstract
 Abstract Abstract
 PDF PDF
 - Objectives
This study aimed to assess the contact tracing outcomes of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron sub-lineages BA.4, BA.5, and BA.2.75 within Republic of Korea, and to generate foundational data for responding to future novel variants.
Methods
We conducted investigations and contact tracing for 79 confirmed BA.4 cases, 396 confirmed BA.5 cases, and 152 confirmed BA.2.75 cases. These cases were identified through random sampling of both domestically confirmed and imported cases, with the goal of evaluating the pattern of occurrence and transmissibility.
Results
We detected 79 instances of Omicron sub-lineage BA.4 across a span of 46 days, 396 instances of Omicron sub-lineage BA.5 in 46 days, and 152 instances of Omicron sub-lineage BA.2.75 over 62 days. One patient with severe illness was confirmed among the BA.5 cases; however, there were no reports of severe illness in the confirmed BA.4 and BA.2.75 cases. The secondary attack risk among household contacts were 19.6% for BA.4, 27.8% for BA.5, and 24.3% for BA.2.75. No statistically significant difference was found between the Omicron sub-lineages.
Conclusion
BA.2.75 did not demonstrate a higher tendency for transmissibility, disease severity, or secondary attack risk within households when compared to BA.4 and BA.5. We will continue to monitor major SARS-CoV-2 variants, and we plan to enhance the disease control and response systems.
- The effectiveness of Paxlovid treatment in long-term care facilities in South Korea during the outbreak of the Omicron variant of SARS-CoV-2
-
Hanul Park, Young Joon Park, Hye Young Lee, Mi Yu, Yeong-Jun Song, Sang Eun Lee, Ji-Joo Lee, Eun-Sol Lee, Yeonjung Kim
-
Osong Public Health Res Perspect. 2022;13(6):443-447. Published online December 23, 2022
-
DOI: https://doi.org/10.24171/j.phrp.2022.0262
-
-
3,592
View
-
216
Download
-
7
Web of Science
-
9
Crossref
-
 Graphical Abstract Graphical Abstract
 Abstract Abstract
 PDF PDF
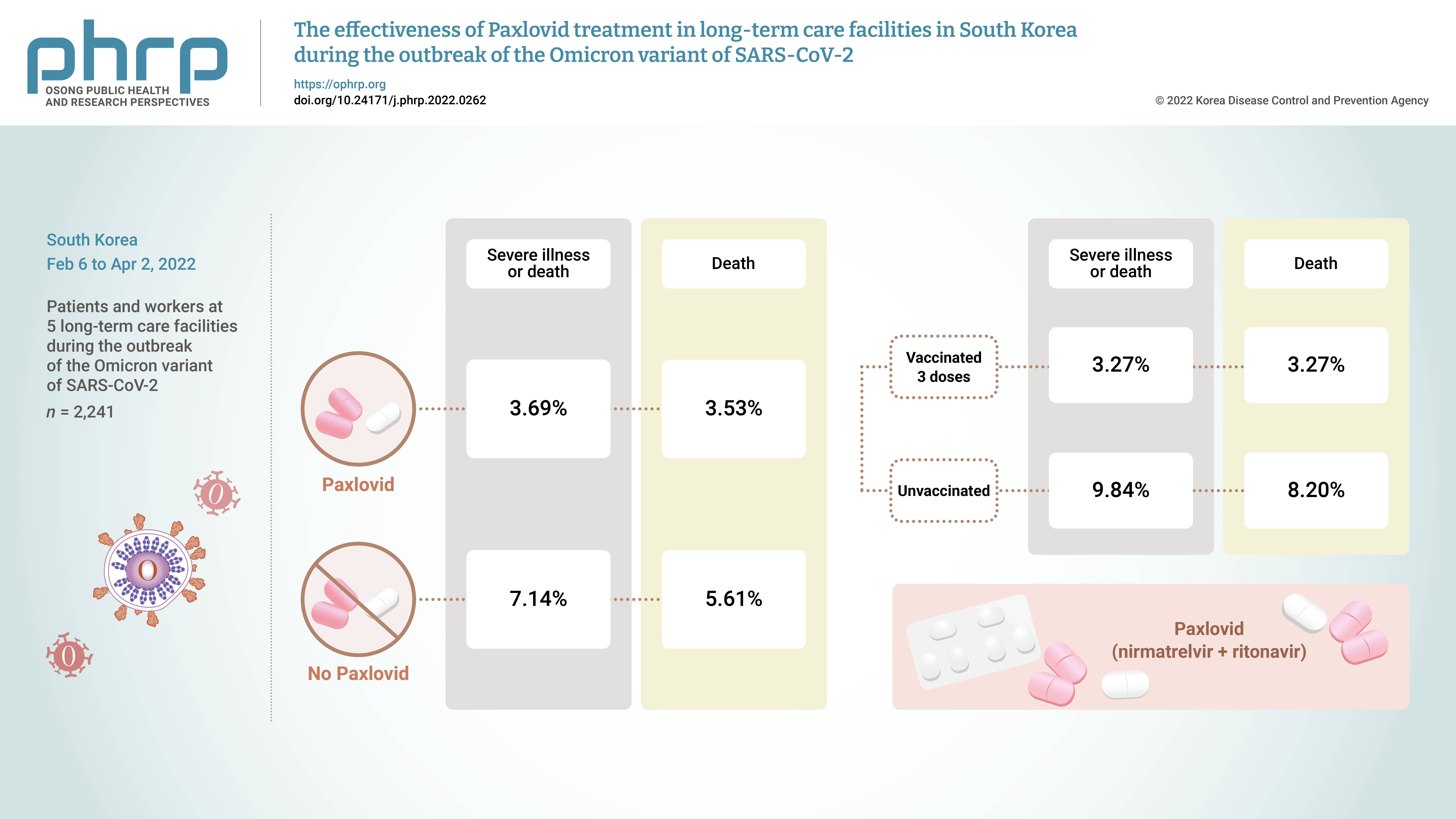 - Objectives
On November 5, 2021, Pfizer Inc. announced Paxlovid (nirmatrelvir +ritonavir) asa treatment method that could reduce the risk of hospitalization or death for patients withconfirmed coronavirus disease 2019 (COVID-19).Methods: From February 6, 2022 to April 2, 2022, the incidence of COVID-19 and the effectsof treatment with Paxlovid were analyzed in 2,241 patients and workers at 5 long-term carefacilities during the outbreak of the Omicron variant of severe acute respiratory syndromecoronavirus 2 in South Korea.Results: The rate of severe illness or death in the group given Paxlovid was 51% lower thanthat of the non-Paxlovid group (adjusted risk ratio [aRR], 0.49; 95% confidence interval [CI],0.24−0.98). Compared to unvaccinated patients, patients who had completed 3 doses of thevaccine had a 71% reduced rate of severe illness or death (aRR, 0.29; 95% CI, 0.13−0.64) and a65% reduced death rate (aRR, 0.35; 95% CI, 0.15−0.79).Conclusion: Patients given Paxlovid showed a lower rate of severe illness or death and alower fatality rate than those who did not receive Paxlovid. Patients who received 3 dosesof the vaccine had a lower rate of severe illness or death and a lower fatality rate than theunvaccinated group.
-
Citations
Citations to this article as recorded by  - Efficacy and safety of antiviral treatments for symptomatic COVID-19 outpatients: Systematic review and network meta-analysis
Meital Zur, Thalia Peselev, Stav Yanko, Victoria Rotshild, Ilan Matok
Antiviral Research.2024; 221: 105768. CrossRef - Clinical Effectiveness of Ritonavir-Boosted Nirmatrelvir—A Literature Review
Sydney Paltra, Tim O. F. Conrad
Advances in Respiratory Medicine.2024; 92(1): 66. CrossRef - Effectiveness of nirmatrelvir‐ritonavir on severe outcomes of COVID‐19 in the era of vaccination and Omicron: An updated meta‐analysis
Sien Ombelet, Diego Castanares‐Zapatero, Fabian Desimpel, Frank Hulstaert, Sabine Stordeur, Dominique Roberfroid
Journal of Medical Virology.2024;[Epub] CrossRef - COVID‐19 infection in patients with haematological malignancies: A single‐centre survey in the latest Omicron wave in China
Xiaolu Zhu, Qian Jiang, Jin Lu, Yuqian Sun, Xiaosu Zhao, Shenmiao Yang, Feifei Tang, Wenjing Yu, Ting Zhao, Xiaohong Liu, Jinsong Jia, Wenbing Duan, Lijuan Hu, Jing Wang, Yang Liu, Nan Peng, Xuelin Dou, Rui Ma, Qiang Fu, Huifang Wang, Kaiyan Liu, Xiaojun
British Journal of Haematology.2023; 202(1): 31. CrossRef - The association mental health of adolescents with economic impact during the COVID-19 pandemic: a 2020 Korean nationally representative survey
Hanul Park, Kang-Sook Lee
BMC Public Health.2023;[Epub] CrossRef - Efficacy and safety of paxlovid (nirmatrelvir/ritonavir) in the treatment of COVID‐19: An updated meta‐analysis and trial sequential analysis
Haokun Tian, Changsen Yang, Tiangang Song, Kechen Zhou, Lequan Wen, Ye Tian, Lirui Tang, Weikai Xu, Xinyuan Zhang
Reviews in Medical Virology.2023;[Epub] CrossRef - Real-World Effectiveness of Nirmatrelvir-Ritonavir and Its Acceptability in High-Risk COVID-19 Patients
Min-Kyung Kim, Kyung-Shin Lee, Sin Young Ham, Youn Young Choi, Eunyoung Lee, Seungjae Lee, Bora Lee, Jaehyun Jeon, BumSik Chin, Yeonjae Kim, Gayeon Kim, Hee-Chang Jang, Jae-Phil Choi, Sang-Won Park
Journal of Korean Medical Science.2023;[Epub] CrossRef - Effectiveness of Molnupiravir Treatment in Patients with COVID-19 in Korea: A Propensity Score Matched Study
Hye Rim Park, Min-Gyu Yoo, Jong Mu Kim, Soon Jong Bae, Hyungmin Lee, Jungyeon Kim
Infection & Chemotherapy.2023; 55(4): 490. CrossRef - Nirmatrelvir combined with ritonavir for preventing and treating COVID-19
Stefanie Reis, Maria-Inti Metzendorf, Rebecca Kuehn, Maria Popp, Ildiko Gagyor, Peter Kranke, Patrick Meybohm, Nicole Skoetz, Stephanie Weibel
Cochrane Database of Systematic Reviews.2023;[Epub] CrossRef
- Clinical outcomes of remdesivir-treated COVID-19 patients in South Korea
-
Mi Yu, Bryan Inho Kim, Jungyeon Kim, Jin Gwack
-
Osong Public Health Res Perspect. 2022;13(5):370-376. Published online October 18, 2022
-
DOI: https://doi.org/10.24171/j.phrp.2022.0138
-
-
2,094
View
-
72
Download
-
2
Web of Science
-
2
Crossref
-
 Abstract Abstract
 PDF PDF
- Objectives
This study analyzed the clinical outcomes of remdesivir treatment in coronavirus disease 2019 (COVID-19) patients in South Korea.
Methods
This retrospective cohort study involved the secondary analysis of epidemiological data. Among patients diagnosed with COVID-19 from July 2, 2020 to March 23, 2021 (12 AM), 4,868 who received oxygen therapy and were released from isolation after receiving remdesivir treatment were assigned to the treatment group, and 6,068 patients who received oxygen therapy but not remdesivir were assigned to the untreated group. The study subjects included children under the age of 19. The general characteristics and severity were compared between the groups. Differences in the time to death and mortality were also compared.
Results
In the untreated group, the hazard ratio [HR] for mortality was 1.59 among patients aged ≥70 years and 2.32 in patients with severe disease in comparison to the treatment group. In a comparison of survival time among patients with severe disease aged ≥70 years, the HR for mortality before 50 days was 2.09 in the untreated group compared to the treatment group.
Conclusion
Patients with remdesivir treatment showed better clinical outcomes in this study, but these results should be interpreted with caution since this study was not a fully controlled clinical trial.
-
Citations
Citations to this article as recorded by  - The Role of Multidimensional Prognostic Index to Identify Hospitalized Older Adults with COVID-19 Who Can Benefit from Remdesivir Treatment: An Observational, Prospective, Multicenter Study
Carlo Custodero, Nicola Veronese, Eva Topinkova, Helena Michalkova, Maria Cristina Polidori, Alberto Cella, Alfonso J. Cruz-Jentoft, Christine A. F. von Arnim, Margherita Azzini, Heidi Gruner, Alberto Castagna, Giovanni Cenderello, Romina Custureri, Tania
Drugs & Aging.2023; 40(7): 643. CrossRef - Remdesivir: A Review in COVID-19
Hannah A. Blair
Drugs.2023; 83(13): 1215. CrossRef
|